Abstract
Recent data suggest that severe cytopenia may be an underestimated adverse effect of CD19+ CAR T-cells. In the current study, we have investigated the incidence of severe cytopenia and its clinical impact after therapy with commercial CD19 CAR-T cell products in patients with large-B-cell lymphoma (LBCL) in Europe. Data on cytopenia were collected via a form designed for the post-authorization studies on CAR-T cell therapy. In this form, occurrence, time of onset and grading of cytopenia were reported. Severe cytopenia was defined as grade 3 (Hb<8g/dl; Neutrophils <1x109/l; Platelets <50x109/l) or grade 4 (Hb<6.5g/dl; Neutrophils <0.5x109/l; Platelets <10x109/l) according to the National Cancer Institute Common Terminology Criteria for Adverse Events
The primary study endpoint was incidence of severe cytopenia in the first 100 days after CAR-T cell infusion. Secondary study endpoints were overall survival, progression-free survival, non-relapse mortality, relapse and infections. For multivariate analyses, we included potential risk factors: year of CAR-T cell infusion, age, sex, previous transplantation, and disease status at CAR-T.
We identified 317 adult patients with LBCL receiving CD19+ CAR-T cell therapy who were reported to the EBMT and had the full dataset needed for this study available. Patients received Axi-Cel (64.2%) or Tisa-Cel (35.8%). The median follow-up was 12.4 months. Most patients received CAR-T cells without having a previous transplantation (76%; neither autologous nor allogeneic). Disease status before CAR-T cell therapy was progressive disease in 83.2%, stable disease in 5.1% and partial/complete remission in 11.7%. Most patients had received either two or three previous lines of therapy. However, 25.6% had four or more lines of previous therapy. When comparing characteristics of patients who developed CART-associated bone marrow aplasia (n=43) with patients who did not (n=274) no significant differences regarding age, sex, Karnofsky score, disease status or history of transplantation were detected.
The cumulative incidence of severe cytopenia was 10.7% at 30 days (95% CI [7.6-14.4]) and 13.6% at 100 days after CAR-T cell infusion (95% CI [10.1-17.6]). The median time from CAR-T cell infusion to onset of severe cytopenia was 15 days (min-max; [IQR] = 1-90; [7-29]). Grade 3 and grade 4 CTCAE cytopenia occurred in 19.3% and 80.7% of patients, respectively. Most patients had either pancytopenia (44%) or isolated neutropenia (48%). Of note, 45.9% suffered from prolonged severe cytopenia without resolution until day +100 after CAR-T cell infusion.
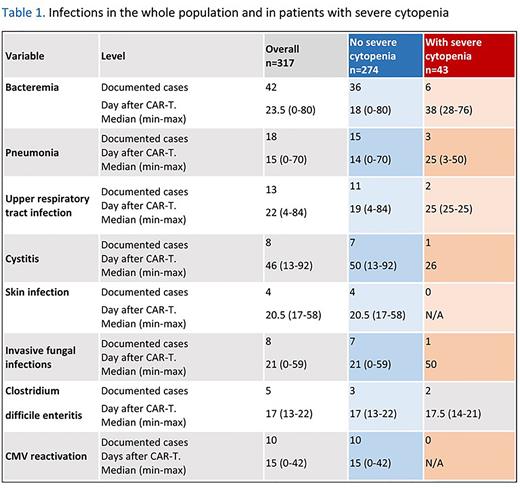
Infections, such as bacteremia, enteritis and cystitis were reported in patients who developed severe cytopenia (Table 1). However, severe cytopenia had no significant impact on overall survival (HR 1.07 [95%CI 0.66-1.73] p=0.78). Of note, patients with severe cytopenia had a lower progression-free survival (HR 1.59 [95%CI 1.07-2.36] p=0.02) and a higher relapse incidence (HR 1.59 [95%CI 1.06-2.39] p=0.025). Due to the low number of patients with non-relapse mortality in the severe cytopenia group (n=3), we were unable to perform multivariate analyses. Outcome graphs are shown in Figure 1.
Our data provide insight on frequency and clinical relevance of severe cytopenia after CAR-T cell therapy for LBCL in the European real world setting.
Disclosures
Penack:MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Therakos: Honoraria; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees; Priothera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Koenecke:Novartis: Membership on an entity's Board of Directors or advisory committees. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Dreger:Novartis: Honoraria; Kite: Honoraria. Bloor:Janssen: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau. Ganser:Jazz Pharmaceuticals: Consultancy; Novartuis: Consultancy; Celgene: Consultancy. Forcade:Sanofi: Other: Travel Support; GSK: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Other: Travel Support, Speakers Bureau; Gilead: Other: Travel Support, Speakers Bureau; MSD: Other: Travel Support. Novak:Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Ideogen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; H & O Communication: Honoraria. Moiseev:Takeda: Honoraria; Jazz: Honoraria; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Schoemans:serEBMT, EUPATI (the European Patient academy): Other: serves regularly as a volunteer for EBMT and occasionally for EUPATI (the European Patient academy); Novartis and the BHS (Belgian Hematological Society): Research Funding; Belgian Hematological Society (BHS): Other: Fees paid to institution; Incyte, Janssen, Novartis , Jazz Pharmaceuticals, Takeda: Other: Personal Fees. Basak:Human Biome Institute: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Saventic Health: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Chabannon:BMS/CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Speakers Bureau; EBMT: Membership on an entity's Board of Directors or advisory committees; TERUMO BCT: Speakers Bureau; MILTENYI BIOTECH: Research Funding; FRESENIUS KABI: Research Funding; JANSSEN PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; BELLICUM PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; SANOFI SA: Honoraria, Research Funding, Speakers Bureau. Sureda:MSD: Honoraria; ROCHE: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; BMS: Consultancy, Honoraria; SANOFI: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau; GILEAD: Consultancy. Averbuch:Takeda: Consultancy, Speakers Bureau; Pfizer: Consultancy; GSK: Speakers Bureau. Glass:Novartis: Honoraria; Roche: Research Funding; BMS: Honoraria; Abbvie: Honoraria; Riemser: Research Funding; Roche: Honoraria; Gilead: Honoraria; Miltenyi: Honoraria; JAZZ: Honoraria. De La Camara:MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Honoraria, Membership on an entity's Board of Directors or advisory committees; MODERNA: Honoraria, Speakers Bureau; ATARA: Honoraria, Speakers Bureau; PFIZER: Honoraria, Speakers Bureau; Pierre Fabre: Honoraria, Membership on an entity's Board of Directors or advisory committees; GILEAD: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal